Lumigan + Applicators
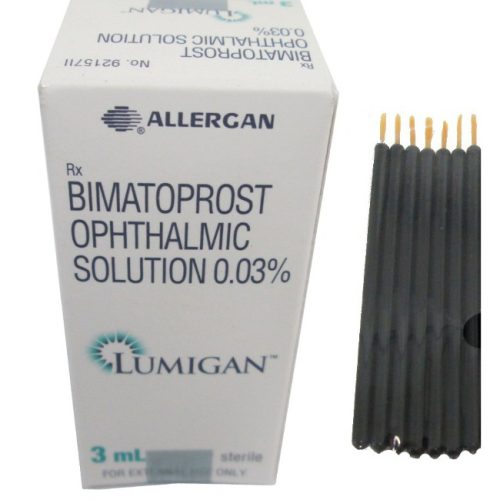
Lumigan + Applicators
- In our pharmacy, you can buy lumigan + applicators without a prescription, with delivery in 5–14 days throughout Australia. Discreet and anonymous packaging.
- Lumigan is used for the treatment of elevated intraocular pressure in open-angle glaucoma and ocular hypertension. The drug is a prostaglandin analogue that increases the outflow of aqueous humour, thereby reducing intraocular pressure.
- The usual dose of lumigan is 1 drop in the affected eye(s) once daily in the evening.
- The form of administration is an ophthalmic solution.
- The effect of the medication begins within 4 hours.
- The duration of action is up to 24 hours.
- Do not consume alcohol.
- The most common side effect is conjunctival hyperemia (red eyes).
- Would you like to try lumigan + applicators without a prescription?
Basic Lumigan + Applicators Information
| INN (International Nonproprietary Name) | Bimatoprost |
|---|---|
| Brand Names Available in Australia | Lumigan |
| ATC Code | S01EE03 |
| Forms & Dosages | Ophthalmic solution (0.01% and 0.03%) |
| Manufacturers in Australia | Allergan |
| Registration Status in Australia | Prescription-only (Rx) |
| OTC / Rx Classification | Prescription only |
Critical Warnings & Restrictions
Before considering Lumigan, it is essential to be aware of certain warnings and restrictions, particularly regarding high-risk groups.
- The elderly, pregnant women, and individuals with chronic diseases may face increased risk of adverse side effects.
- Consulting healthcare providers before use is crucial for these populations.
In Australia, Lumigan is classified as a prescription-only medication. It is regulated by the TGA (Therapeutic Goods Administration), meaning that you must obtain a doctor's approval to use it. This helps ensure proper monitoring and safety during use.
Patients should also consider the potential impact of Lumigan on daily activities. Some individuals might experience side effects that could impair their ability to drive or operate machinery. Prompt consultation with a healthcare professional is advised if any such symptoms arise.
Q&A — “Can I Drive After Taking It in Australia?”
It is essential to ascertain how Lumigan affects you personally before driving. If you experience any side effects like blurred vision or eye discomfort, it is advisable to avoid driving until these effects pass.
Usage Basics
Bimatoprost is marketed as Lumigan in Australia and is available in varying concentrations of 0.01% and 0.03%. The availability of these doses allows healthcare providers to tailor treatment to individual needs.
As a prescription medicine, Lumigan has received TGA approval and is listed under the Pharmaceutical Benefits Scheme (PBS). This regulatory framework ensures that the medication is dispensed responsibly through pharmacies and hospitals across Australia.
Dosing Guide
For managing glaucoma, Lumigan is typically administered as 1 drop in the affected eye(s) once daily in the evening.
Strict adherence to the dosing schedule is crucial for it to be effective. Missing doses can lead to increased intraocular pressure, limiting the medication's efficacy.
Special considerations are necessary for chronic conditions, particularly for the elderly. Always consult healthcare professionals for advice tailored to individual health needs.
Q&A — “What If I Miss a Dose?”
If a dose is missed, it should be instilled as soon as remembered. However, if it is close to the next scheduled dose, it is best to skip the missed dose and continue with the regular schedule. Under no circumstances should you double drop.
Interaction Chart
While using Lumigan, it is important to be cautious about certain foods and drinks. Alcohol and caffeine can exacerbate side effects or diminish the therapeutic efficacy of the medication.
If taking other medications, it is advisable to discuss potential interactions with a healthcare provider. This will help in managing any risks associated with combining treatments.
User Reports & Trends
Feedback from Australian patients on forums and other platforms like ProductReview suggests differing experiences with Lumigan in terms of both its efficacy and side effects. Many users report positive outcomes, particularly regarding eyelash growth, though side effects can vary.
Understanding these user reports can help prospective patients set realistic expectations. It's crucial to consider both benefits and downsides and engage in open discussions with healthcare providers about any concerns.
Access & Purchase Options
Getting access to effective treatments like Lumigan is vital, especially for those managing glaucoma and ocular hypertension. Thankfully, availability is broad across Australia.
- National chains: Major pharmacy chains such as Chemist Warehouse, Priceline, and TerryWhite Chemmart stock Lumigan. This extensive distribution ensures that both urban and rural patients can easily obtain their much-needed medications.
Furthermore, online pharmacies have started to play a pivotal role. Thanks to the rise of telehealth services, patients now have the option to receive e-prescriptions. This is especially beneficial for those living in remote areas, as it allows them to access Lumigan while still adhering to PBS guidelines. The convenience of ordering online means that getting Lumigan can be done from the comfort of home, ensuring timely treatment without the need for lengthy wait times.
Mechanism & Pharmacology
Understanding how Lumigan works can inspire confidence in its use.
- Simplified explanation: Bimatoprost, the active ingredient in Lumigan, functions by relaxing the eye's drainage canals. This action allows more fluid to flow out of the eye, ultimately reducing intraocular pressure.
In clinical terms, Bimatoprost is classified as a prostaglandin analogue. This classification is significant because prostaglandin analogues are known for their effectiveness in treating glaucoma. Their mechanism enhances the outflow of aqueous humour, which is essential for managing eye pressure. Understanding this pharmacological action is crucial in discussing treatment options, as it highlights why Lumigan is a popular choice among healthcare providers in managing ocular conditions.
Indications & Off-Label Uses
Lumigan has established itself as a reliable treatment option with a clear indication for use.
- Approved indications by TGA: The primary use of Lumigan is for managing elevated intraocular pressure in patients with open-angle glaucoma and ocular hypertension.
However, it is also known for its off-label use in cosmetic applications. Many individuals have begun using Lumigan for eyelash enhancement. While the results can be dramatic, it’s essential to communicate the associated risks effectively, particularly regarding the proper use of applicators. Misuse can lead to unintended hair growth in areas other than the eyelids, highlighting the importance of adhering to guidance from healthcare professionals.
Key Clinical Findings
Recent research has brought new insights into Lumigan's effectiveness.
- Major Australian and international studies (2022–2025): Various studies have shown that Lumigan is not only effective but also boasts a robust safety profile, comparing favourably against other treatments such as Latanoprost.
An analysis of these findings indicates that patients often experience better outcomes with Lumigan, making it a preferred choice among practitioners. Such analyses are crucial for healthcare providers when determining the best treatment regime for individuals struggling with intraocular pressure issues.
Alternatives Matrix
When considering treatment options, comparing alternatives can be invaluable.
A PBS-listed alternatives comparison table for Lumigan includes:
| Brand Name | ATC Code | Principal Use |
|---|---|---|
| Xalatan (Latanoprost) | S01EE01 | Glaucoma, OHT |
| Travatan (Travoprost) | S01EE04 | Glaucoma, OHT |
| Taflotan (Tafluprost) | S01EE05 | Glaucoma, OHT |
| Ganfort (Bimatoprost + Timolol) | S01ED51 | Combination therapy |
This comparison sheds light on the prescribing patterns and effectiveness of Lumigan relative to its competitors.
To aid patients in making informed decisions, here’s a quick pros and cons checklist:
- Pros: Effective in lowering intraocular pressure, easy to administer, and widely available.
- Cons: Some potential side effects, off-label risks with cosmetic use, and the necessity of applicators for eyelash enhancement.
Common Questions
Patients often have pressing questions about Lumigan, particularly surrounding side effects, costs, and alternative therapies. Understanding these concerns is crucial for pharmacists in Australia. Common side effects include conjunctival hyperemia, eye irritation, and changes in eyelash growth. Most individuals find these manageable; however, the potential for increased iris pigmentation can be a worry. Cost is another concern, especially for those without health coverage. Lumigan is subsidised under the Pharmaceutical Benefits Scheme (PBS), significantly reducing expenses for eligible patients. Some might also ask if there are alternative therapies for glaucoma management. While Lumigan is effective, other options such as Latanoprost or Travoprost may be comparable, depending on the individual. Patients should discuss these alternatives with their pharmacists to find the best fit for their needs. Such dialogues ensure patients remain informed and confident in their treatment choices.
Suggested Visual Content
Infographics can significantly enhance understanding. Consider creating visuals that outline the following:
- PBS Pricing Structures: An overview of costs associated with Lumigan and patient subsidies.
- Pharmacy Access in Australia: A map detailing how pharmaceutical services are distributed across urban and rural areas.
- Usage Guidelines for Lumigan: An instructional visual on the correct application method, especially when using applicators for cosmetic purposes.
Registration & Regulation
Lumigan is strictly regulated in Australia, ensuring that it meets TGA (Therapeutic Goods Administration) standards for safety and efficacy. This process includes comprehensive assessments of clinical data before approval. Patients can be confident that they are receiving a medication that has been thoroughly evaluated. Under the PBS, Lumigan receives a subsidy, allowing eligible individuals to access it at a lower cost. This is particularly beneficial for those managing chronic conditions such as glaucoma. Eligibility for subsidies often depends on specific criteria, including medical necessity and income thresholds. Navigating these options can be complex, but consulting with a pharmacist can help clarify eligibility and affordability.
Storage & Handling
Storing Lumigan properly, especially in Australia's varied climate, is essential to maintain its efficacy. Products should ideally be kept at temperatures between 15–25°C, as exposure to heat and light can compromise the solution. For households, keeping the medication in a cool, dark place, such as a cupboard, can prevent degradation. Pharmacies also have a responsibility to ensure cold-chain handling of Lumigan, particularly when distributing to rural areas. Transporting these products while keeping them at the recommended temperatures can be challenging, but it is vital for ensuring patient safety and product integrity. Pharmacists should consistently monitor storage conditions to avoid temperature fluctuations and ensure customers receive quality medications.
Guidelines for Proper Use
Effective communication is key in pharmacist counselling regarding Lumigan usage. Pharmacists should provide clear instructions on how to administer drops properly, including the correct positioning for eye drops and how to use applicators when prescribed for eyelash enhancement. Guidelines from health authorities stress the importance of adherence to treatment regimens, particularly for glaucoma, as missed doses can have serious implications for intraocular pressure management. Patients should be advised to monitor for any adverse effects and report them immediately, especially eye irritation or changes in vision. A personal approach, where pharmacists check in with patients about their treatment progress, can foster better relationships and interventions for any concerns that may arise during use.
| City | Region | Delivery Time |
|---|---|---|
| Sydney | New South Wales | 5–7 days |
| Melbourne | Victoria | 5–7 days |
| Brisbane | Queensland | 5–7 days |
| Perth | Western Australia | 5–7 days |
| Adelaide | South Australia | 5–7 days |
| Hobart | Tasmania | 5–9 days |
| Canberra | Australian Capital Territory | 5–9 days |
| Gold Coast | Queensland | 5–9 days |
| Newcastle | New South Wales | 5–9 days |
| Cairns | Queensland | 5–9 days |
| Wollongong | New South Wales | 5–9 days |
| Geelong | Victoria | 5–9 days |
| Sunshine Coast | Queensland | 5–9 days |
| Townsville | Queensland | 5–9 days |
| Ballarat | Victoria | 5–9 days |