Spiriva
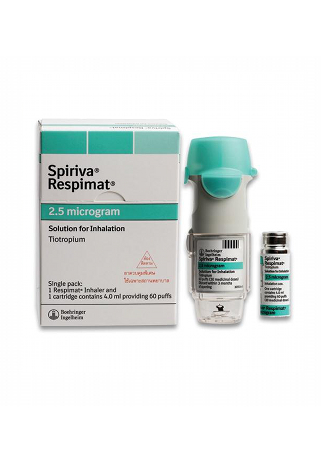
Spiriva
- In our pharmacy, you can buy Spiriva without a prescription, with delivery in 5–14 days throughout Australia. Discreet and anonymous packaging.
- Spiriva is used for the long-term management of chronic obstructive pulmonary disease (COPD) and asthma. It works as an anticholinergic bronchodilator, helping to open airways by blocking specific receptors.
- The usual dose for COPD is 18 mcg via the HandiHaler once daily, and for asthma, it is 1.25 mcg (2 puffs) once daily using the Respimat inhaler.
- The form of administration is through inhalation, either as a powder capsule or a liquid solution.
- The effect of the medication begins within 30 minutes.
- The duration of action is up to 24 hours.
- It is advisable to avoid alcohol while using this medication.
- The most common side effect is dry mouth.
- Would you like to try Spiriva without a prescription?
Basic Spiriva Information
- INN (International Nonproprietary Name): Tiotropium bromide
- Brand names available in Australia: Spiriva HandiHaler, Spiriva Respimat
- ATC Code: R03BB04
- Forms & dosages: Inhalation powder capsules, inhalation solutions
- Manufacturers in Australia: Boehringer Ingelheim
- Registration status in Australia: Registered and TGA approved
- OTC / Rx classification: Prescription only (Rx)
Critical Warnings & Restrictions
When considering Spiriva, it's crucial to be aware of specific precautions, especially for high-risk groups. The elderly, pregnant individuals, and those suffering from chronic illnesses need to take extra care, particularly because of the potential for anticholinergic effects. These effects can be particularly marked in those who already have compromised systems.
High-Risk Groups
If you belong to any high-risk category, it's essential to consult with a healthcare professional to determine the suitability of Spiriva for your needs. Anticholinergic effects may pose heightened risks, such as increased heart rate and blurred vision, in these populations.
Interaction with Activities
Driving and workplace safety while using Spiriva is another critical aspect to consider. The Australian laws highlight the need for caution regarding activities requiring full attention, as some users may experience side effects like dizziness.
Q&A — “Can I drive after taking it in Australia?”
Generally, if experiencing side effects like dizziness after taking Spiriva, it's best to avoid driving until you know how you individually react to the medication. Always prioritise safety above all else.
Usage Basics
Spiriva contains the active ingredient tiotropium bromide and is available in two primary forms in Australia: the Spiriva HandiHaler, delivering an 18 mcg inhalation powder, and the Spiriva Respimat, which provides an inhalation solution in two strengths: 2.5 mcg and 1.25 mcg. Both forms are designed to assist in the management of COPD and asthma.
Legal Classification
Spiriva is classified as a prescription-only medication in Australia, having received approval from the Therapeutic Goods Administration (TGA). The prescription status confirms its intended use within medical guidelines for treating respiratory diseases, ensuring it is appropriately managed by healthcare practitioners.
Dosing Guide
Regular dosing of Spiriva is crucial for effective management of COPD and asthma. According to PBS reference guidelines, the recommended dosage involves inhaling one capsule (18 mcg) daily with the HandiHaler or, for those using the Respimat, two puffs delivering 2.5 mcg each, also once daily.
Standard Regimens
The dosage may need adjustment based on specific patient requirements. It's particularly important for children, the elderly, and those with liver or kidney impairment to have tailored dosing plans to ensure safety and efficacy.
Adjustments for Comorbidities
Be especially vigilant when administering Spiriva to children or elderly patients. For children, Spiriva Respimat is approved for those aged 6 and older, while HandiHaler is not recommended. In cases of kidney impairment, close monitoring is advised.
Q&A — “What if I miss a dose?”
If you happen to miss a dose of Spiriva, do not double up to compensate. Simply wait until your next scheduled dose and continue as usual. Consistency is key in maintaining the benefits of treatment without risking potential overdoses.
Interaction Chart
Interactions with certain food and beverages can affect the efficacy of Spiriva. For instance, collagen-rich beverages may be avoided to ensure the drug works effectively within your system. Additionally, caffeine and alcohol should be moderated while using this medication.
Food and Drinks
The impact of food interactions, especially with substances like alcohol and coffee, cannot be overlooked. Both can amplify side effects, and advising individuals to consume these substances with caution while on Spiriva is vital.
Common Drug Conflicts
Several medications might conflict with Spiriva's effectiveness. Checking with a pharmacist or healthcare provider about current prescriptions is advisable to avoid adverse interactions.
User Reports & Trends
Feedback from Australian patients, available on platforms like ProductReview and various health forums, indicates a mixed bag of experiences. Many individuals report positive outcomes regarding Spiriva's effectiveness in managing symptoms of COPD and asthma, emphasising its ease of use. However, common concerns usually revolve around side effects and managing the initial adjustment period. Continuous evaluation remains essential, and consulting healthcare professionals for any adverse reactions is encouraged that could specifically relate to Spiriva.
Access & Purchase Options
Spiriva can be found in major pharmacy chains such as Chemist Warehouse, Priceline, and TerryWhite across Australia. Their extensive networks make accessing Spiriva relatively easy.
National Chains
Shoppers can conveniently find Spiriva at national chains, which stock both the HandiHaler and Respimat forms. Be sure to check with pharmacists for the availability of specific strengths and forms.
Online Pharmacies and Telehealth E-Prescriptions
The growth of online pharmacies and telehealth services has revolutionised access to medications like Spiriva. By enabling e-prescriptions, individuals can receive their medications without unnecessary delays, enhancing their treatment schedules and overall management of conditions.
Mechanism & Pharmacology
Simplified Explanation
Spiriva, known generically as tiotropium, is an anticholinergic bronchodilator that works mainly by blocking the action of acetylcholine in the lungs, leading to relaxation and widening of the airways. This allows easier breathing for individuals with chronic obstructive pulmonary disease (COPD) and asthma. By targeting the muscarinic receptors, Spiriva reduces bronchoconstriction and helps to keep the airways open longer than other similar medications.
Clinical Terms
The term "anticholinergic" refers to substances that inhibit the action of acetylcholine, a neurotransmitter that can cause the muscles around the airways to constrict. "Bronchodilator" describes any drug that dilates the airways; in Spiriva's case, it specifically works on certain receptors to achieve this effect. "Muscarinic antagonist" is a type of anticholinergic that specifically blocks muscarinic receptors, thus preventing airway narrowing.
Indications & Off-Label Uses
Approved Indications by TGA
Spiriva is primarily prescribed for the maintenance treatment of COPD in adults and for asthma in children aged six and older. Eligible patients typically have persistent symptoms and experience frequent exacerbations. For effective use, healthcare providers take into account the severity of the condition, previous therapies tried, and individual patient responses to treatment.
Off-Label Uses in Australian Clinical Practice
In addition to its approved uses, Spiriva has been employed in the management of certain off-label conditions, such as chronic asthma in adults where standard first-line treatments are inadequate. Australian guidelines suggest that Spiriva may be prescribed as part of a broader treatment regimen, particularly for those with both asthma and COPD features, helping to improve overall lung function and reduce exacerbation rates.
Key Clinical Findings
Recent studies from Australia and internationally between 2022 and 2025 have highlighted Spiriva's long-term efficacy in controlling asthma and COPD symptoms. Research indicates significant reductions in exacerbations and hospitalisations due to respiratory distress among patients on Spiriva compared to controls. Notably, a 2023 study revealed that patients experienced improved quality of life metrics and lung function test results after six months of consistent Spiriva therapy. Safety profiles remained consistent, with manageable side effects. These findings underscore Spiriva's role as a vital component in managing chronic respiratory diseases and maintaining patient health over time.
Alternatives Matrix
PBS-Listed Alternatives Comparison Table
| Medication | ATC Code | Formulation | Dose |
|---|---|---|---|
| Spiriva (Tiotropium) | R03BB04 | Inhalation powder | 18 mcg once daily |
| Aclidinium | R03BB02 | Inhalation powder | 400 mcg twice daily |
| Glycopyrronium | R03BB03 | Inhalation powder | 50 mcg once daily |
| Umeclidinium | R03BB06 | Inhalation powder | 62.5 mcg once daily |
Pros and Cons Checklist
- Pros:
- Effective long-term control of COPD and asthma symptoms.
- Once-daily dosing enhances compliance.
- Low incidence of systemic side effects compared to some alternatives.
- Cons:
- May cause dry mouth and constipation as common side effects.
- Not suitable for acute asthma attacks.
- Potential risk of urinary retention in predisposed individuals.
Common Questions
During pharmacy consultations, several questions frequently arise regarding Spiriva. Key FAQs include:
- Do side effects of Spiriva go away? Many users find that side effects diminish over time as their body adjusts.
- How to use Spiriva effectively? Proper technique in using Spiriva, whether HandiHaler or Respimat, is crucial for optimal delivery and efficacy.
- What if a dose is missed? It’s advised to skip missed doses and continue with the next scheduled dose. Doubling up is not recommended.
- Is Spiriva a steroid? No, Spiriva is not a steroid; it is an anticholinergic bronchodilator.
Suggested Visual Content
To effectively inform both patients and healthcare professionals about Spiriva (tiotropium), a variety of visual content formats can be incredibly helpful. For instance:
- Infographics illustrating PBS pricing can clarify the cost implications for patients.
- Pharmacy network maps demonstrating where Spiriva is available can assist patients in locating nearby pharmacies.
- Clear dosage instructions with visual aids can guide patients on how to use the Spiriva HandiHaler and Respimat inhalers correctly.
Registration & Regulation
TGA Approval
Spiriva is subject to regulation by the Therapeutic Goods Administration (TGA) in Australia. It requires stringent compliance with standards for quality, safety, and efficacy. Approval ensures that Spiriva meets the necessary benchmarks for its intended use in managing chronic obstructive pulmonary disease (COPD) and asthma. Health professionals can trust the product's integrity, knowing it has been thoroughly evaluated by TGA.
PBS Subsidy Details
The Pharmaceutical Benefits Scheme (PBS) offers subsidies for Spiriva, significantly reducing costs for eligible patients. Individuals diagnosed with COPD or asthma may qualify, leading to a lower out-of-pocket expense. The exact subsidy amount can vary based on factors like individual patient circumstances and government regulations. For patients, understanding these details can ease financial burdens in managing their conditions effectively.
Storage & Handling
Household Storage in Australian Climate
In Australia, maintaining the efficacy of Spiriva products requires careful attention to storage conditions. They should ideally be stored below 25°C in a dry environment, away from direct sunlight and moisture. Keeping the inhalers in their original packaging until use can also help preserve their quality. This is especially crucial given Australia's varied climate, where extreme heat can affect medication integrity.
Cold-Chain Handling for Pharmacies
For pharmacies, adhering to cold-chain requirements is essential in ensuring that Spiriva medications are stored correctly. This includes maintaining a temperature-controlled environment to prevent degradation of the active ingredients. Regular temperature checks and proper storage practices help pharmacies guarantee that they are dispensing safe, effective medications to customers.
Guidelines for Proper Use
Australian Pharmacist Counselling Style
Pharmacists play a pivotal role in guiding patients on the correct use of Spiriva. Their counselling typically includes:
- Demonstrating how to use the Spiriva HandiHaler and Respimat inhalers, highlighting step-by-step techniques.
- Discussing common side effects, such as dry mouth and how to manage them effectively.
- Encouraging patients to maintain adherence to their prescribed regimen and explaining the significance of consistent use.
Patient Advice from PBS and National Health Authorities
Recommendations from PBS and health authorities stress the importance of using Spiriva strictly as directed. It’s essential to inform patients that this medication is not intended for acute relief; instead, it serves as a maintenance therapy for chronic conditions like COPD and asthma. Regular follow-ups are advisable to monitor effectiveness and adjust dosages if necessary. Patients should also be aware of potential interactions and discuss any other medications they are taking with their healthcare providers. These insights ensure safe and effective use of Spiriva.
| City | Region | Delivery Time |
|---|---|---|
| Sydney | NSW | 5–7 days |
| Melbourne | VIC | 5–7 days |
| Brisbane | QLD | 5–7 days |
| Perth | WA | 5–7 days |
| Adelaide | SA | 5–7 days |
| Hobart | TAS | 5–9 days |
| Cairns | QLD | 5–9 days |
| Canberra | ACT | 5–7 days |
| Gold Coast | QLD | 5–9 days |
| Geelong | VIC | 5–9 days |
| Newcastle | NSW | 5–9 days |
| Sunshine Coast | QLD | 5–9 days |
| Wollongong | NSW | 5–9 days |
| Townsville | QLD | 5–9 days |
| Launceston | TAS | 5–9 days |
| Ballarat | VIC | 5–9 days |