Tiova Inhaler
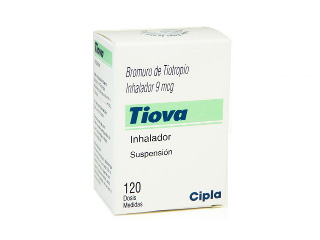
Tiova Inhaler
- In our pharmacy, you can buy Tiova inhaler without a prescription, with delivery in 5–14 days throughout Australia. Discreet and anonymous packaging.
- Tiova inhaler is intended for the treatment of chronic obstructive pulmonary disease (COPD) and asthma. The drug is a long-acting muscarinic antagonist (LAMA) that works by relaxing the muscles in the airways to improve breathing.
- The usual dosage of Tiova inhaler is 2 puffs (9 mcg each) once daily.
- The form of administration is a metered-dose inhaler.
- The effect of the medication begins within 30 minutes.
- The duration of action is up to 24 hours.
- Do not consume alcohol while using this medication.
- The most common side effect is dry mouth.
- Would you like to try Tiova inhaler without a prescription?
Basic Tiova Inhaler Information
- INN (International Nonproprietary Name): Tiotropium Bromide
- Brand names available in Australia: Tiova
- ATC Code: R03BB04
- Forms & dosages: Metered inhaler (9 mcg per actuation)
- Manufacturers in Australia: Cipla (India)
- Registration status in Australia: TGA approved
- OTC / Rx classification: Prescription only
Critical Warnings & Restrictions
Patients considering the use of a Tiova inhaler need to be aware of some critical warnings and restrictions associated with this medication.
High-risk Groups (Elderly, Pregnancy, Chronic Illness)
Those in high-risk groups, including elderly individuals, pregnant and breastfeeding women, and patients with chronic illnesses, should approach the Tiova inhaler with caution. The inhaler contains Tiotropium Bromide, an anticholinergic medication that can affect everyone differently. Elderly patients are often more sensitive to medications, including Tiova, and may experience heightened side effects. Pregnant and breastfeeding women should consult their healthcare providers to assess the risks and benefits before starting treatment.
Additionally, individuals with chronic respiratory conditions or other comorbid conditions must seek medical advice to determine the appropriateness of utilizing the Tiova inhaler as part of their treatment plan. Prioritising thorough discussions with healthcare professionals is key to ensuring safety while using this inhaler.
Interaction With Activities (Driving, Workplace Safety Under Australian Law)
Tiova inhaler may have side effects that could impair driving or operating machinery safely. Common symptoms like dizziness or fatigue should not be overlooked, especially for those who drive or work in safety-sensitive environments. Under Australian law, medication use can impact workplace safety regulations, so it’s crucial to check for potential side effects that could pose risks while performing tasks that require full attention.
Q&A — “Can I Drive After Taking It In Australia?”
1. Can Tiova affect my ability to drive?
It has the potential to affect your ability to drive, particularly if side effects like dizziness occur.
2. What should I consider regarding driving post-medication?
Monitor how you feel after taking Tiova. If you experience any unusual symptoms, refrain from driving until you’ve consulted with your doctor.
Usage Basics
The Tiova inhaler, or Tiotropium Bromide, is a well-recognised treatment for chronic respiratory conditions. Understanding its basic usage guides is essential for effective management.
INN, Brand Names Available In Australia
As previously mentioned, Tiotropium Bromide is the International Nonproprietary Name (INN) for the active ingredient in the Tiova inhaler. While Tiova is the primary brand available in Australia, it is crucial to note the packaging details to ensure correct usage of the metered-dose inhaler, typically 9 mcg per actuation.
Legal Classification (TGA-approved, PBS-listed)
Tiova has received approval from the Therapeutic Goods Administration (TGA) for use in managing chronic obstructive pulmonary disease (COPD) and asthma. Additionally, it is listed on the Pharmaceutical Benefits Scheme (PBS), making it more accessible for Australian patients who require this essential inhaler as part of their treatment regimen.
Dosing Guide
When using Tiova inhaler, understanding the proper dosing regimen is critical for achieving optimal health outcomes.
Standard Regimens (PBS Reference Dosing)
For adults managing COPD and asthma, the recommended dose for Tiova is two puffs (totaling 18 mcg) once daily. When using the inhalation capsule form, the dosage remains the same—administer one 18 mcg capsule using the specified inhalation device.
Adjustments For Comorbidities
Dosage adjustments may be necessary for specific populations:
- Children: Tiova is generally not recommended for children under six years, while those aged six and older can typically use the standard adult dosages.
- Elderly: While no specific dosage adjustment is required, caution should be exercised as older patients may experience more pronounced effects.
- Liver or Kidney Impairment: Although no standard adjustments are mandated, close monitoring is recommended for patients with moderate to severe renal impairment.
Q&A — “What If I Miss A Dose?”
1. How should I react if I forget a dose?
Take the missed dose as soon as you remember, unless it's close to your next scheduled dose—then, skip the missed dose.
2. What happens if I take an extra dose?
If you accidentally take an extra dose, seek medical advice and monitor for symptoms of overdose.
Interaction Chart
Understanding potential interactions with food and medications enhances the safety of Tiova inhaler use.
Food And Drinks (Alcohol, Coffee, Australian Diet Context)
Certain dietary content can influence how Tiova works. In particular, alcohol should be limited since it can exacerbate side effects like dizziness. Additionally, high caffeine intake may also pose risks, so moderation is advised.
Common Drug Conflicts
Patients should be aware of possible drug interactions when using Tiova inhaler alongside other medications. This is particularly relevant for those who manage chronic illnesses with multiple prescriptions. Always consult a pharmacist or doctor to ensure that no adverse interactions occur.
User Reports & Trends
Feedback on the Tiova inhaler from Australian review sites like ProductReview highlights various user experiences.
Trends indicate a generally positive reception, with many users reporting improved lung function and fewer symptoms. However, some challenges, such as initial difficulty in using the inhaler correctly, have been noted.
Overall satisfaction levels appear to be high, particularly among those who have found an effective management strategy for their chronic respiratory conditions using Tiova inhaler.
Access & Purchase Options
National chains (Chemist Warehouse, Priceline, TerryWhite)
Tiova inhaler is readily available at major pharmacy chains across Australia, making access easy for patients requiring management of chronic lung diseases. Outlets like Chemist Warehouse, Priceline, and TerryWhite serve as reliable sources for purchasing Tiova without the hassle of long waiting times. These pharmacies understand the importance of patient access and often keep a well-stocked inventory. Having Tiova available on their shelves not only improves accessibility for urban dwellers but also provides support for individuals living in rural areas where health services may be limited.
Online pharmacies and telehealth e-prescriptions
In today’s digital age, obtaining Tiova inhaler through online pharmacies is a breeze. The convenience of telehealth services facilitates e-prescriptions, allowing patients to receive their medications without the need for in-person visits. This is particularly beneficial for those in rural locations who may face challenges in accessing traditional pharmacy services. Patients can connect with healthcare professionals remotely to discuss their conditions, and upon receiving an e-prescription, they can easily order Tiova online. This online accessibility not only breaks down geographical barriers but also enhances medication adherence by ensuring that treatment is readily available.
Mechanism & Pharmacology
Simplified explanation
The active ingredient in Tiova inhaler, Tiotropium Bromide, acts as a long-acting muscarinic antagonist (LAMA). As a bronchodilator, it relaxes the muscles around the airways, making it easier to breathe. This mechanism helps prevent bronchial constriction, allowing for improved airflow in patients suffering from chronic obstructive pulmonary disease (COPD) or asthma. The result is not just relief from symptoms, but a significant improvement in the overall quality of life for those managing these conditions.
Clinical terms
Understanding a few clinical terms can enhance the user's experience with the Tiova inhaler:
- Bronchodilator: A medication that opens up the airways in the lungs.
- LAMA: Long-acting muscarinic antagonist, a class of medication for treating respiratory diseases.
- Maintenance therapy: Long-term treatment aimed at controlling chronic conditions.
Indications & Off-Label Uses
Approved indications by TGA
The Therapeutic Goods Administration (TGA) approves Tiova inhaler for managing serious respiratory conditions, primarily chronic obstructive pulmonary disease (COPD) and asthma in patients aged over six years. By providing symptomatic relief and improving lung function, Tiova assists patients in leading more active and fulfilling lives. Its status as a prescription-only medication ensures that individuals receive appropriate medical guidance and support.
Off-label uses in Australian clinical practice
While Tiova is primarily indicated for COPD and asthma, some healthcare professionals may prescribe it off-label in specific scenarios such as for patients who experience persistent respiratory symptoms despite other treatments. This can include conditions like chronic bronchitis or even acute exacerbations in select cases. However, it's essential for patients to discuss any off-label usage with their healthcare provider to ensure safety and efficacy.
Key Clinical Findings
Recent research has confirmed Tiova inhaler's effectiveness and safety, reinforcing its role in treating chronic lung conditions. Studies conducted from 2022 to 2025, both in Australia and internationally, have shown that Tiova significantly improves lung function and patient-reported outcomes. This solid data supports its longstanding use as a preferred maintenance therapy for patients with COPD and asthma, highlighting the inhaler as a vital option in respiratory health management. Health professionals appreciate these insights as they guide treatment plans tailored to each patient's unique needs.
Alternatives Matrix
PBS-listed alternatives comparison table
| Brand | Active Ingredient | Dosage Form | Notes |
|---|---|---|---|
| Tiova | Tiotropium Bromide | Inhaler (9 mcg) | Long-acting bronchodilator |
| Spiriva | Tiotropium Bromide | Handihaler (18 mcg) | Market leader, similar action |
| Incruse Ellipta | Umeclidinium | Dry powder inhaler | Another LAMA option |
Pros and cons checklist
When considering Tiova versus its alternatives, it's crucial to weigh the pros and cons:
- Pros:
- Once-daily dosage simplifies treatment.
- Proven efficacy in managing COPD and asthma.
- Wide availability through pharmacies and online.
- Cons:
- Not suitable for immediate relief during acute attacks.
- Potential side effects, such as dry mouth or throat irritation.
- Requires proper inhalation technique for maximum effectiveness.
Common Questions
Consulting about the Tiova inhaler often leads to several recurring queries among Australians. Many patients want to know if they can buy the Tiova inhaler without a prescription, as it's sometimes available without one at pharmacies. Another frequent question involves the appropriate dosage—how many puffs are necessary for optimal efficacy? Users often inquire about side effects, especially concerning dry mouth, which is the most common. Some are curious about whether Tiova can be used with a spacer device to improve inhalation. The question of storage is also prominent, particularly regarding how to store the inhaler efficiently in Australia's varying humidity and heat. Lastly, patients seek advice about the difference between Tiova and its competitors like Spiriva, and whether the PBS subsidises the Tiova inhaler, impacting their out-of-pocket costs.
Suggested Visual Content
Visual content can significantly enhance understanding of the Tiova inhaler. Consider developing an infographic detailing the PBS prices for the Tiova inhaler, making it easier for patients to comprehend the financial aspects. Another idea is to create a visual representation of pharmacy networks where the inhaler is available, allowing users to locate their nearest pharmacies efficiently. Additionally, a step-by-step infographic explaining proper inhalation techniques for using the Tiova inhaler can be incredibly beneficial. Not only does this guide users in achieving the best results, but it also addresses common mistakes made while administering medication.
Registration & Regulation
TGA approval
The Tiova inhaler, containing Tiotropium Bromide, has undergone rigorous evaluation and was approved by the Therapeutic Goods Administration (TGA) in Australia. The approval process ensured that Tiova meets safety, quality, and efficacy standards for patients with respiratory conditions, such as COPD and asthma. Registered as a prescription medication, ongoing assessments are conducted by the TGA to monitor its performance and safety post-approval, thus ensuring public safety in its use.
PBS subsidy details
The Pharmaceutical Benefits Scheme (PBS) provides a subsidy for Tiova, making it more accessible for patients in Australia. Under PBS guidelines, patients may only need to pay a fraction of the cost, significantly reducing financial burden. This subsidy is vital for those managing chronic conditions, allowing for consistent and affordable access to essential medications. The precise amount paid by the patient can vary depending on their overall health plan. Consequently, it’s recommended that users discuss their eligibility and the financial implications with their pharmacists.
Storage & Handling
Household storage in Australian climate (heat/humidity)
Storing the Tiova inhaler correctly is crucial, especially considering Australia’s diverse climate. In general, the inhaler should be kept in a cool, dry place, away from direct sunlight and moisture. It’s best to store the inhaler below 30°C to maintain efficacy. Bathrooms or kitchens may not be the best choices due to humidity; instead, consider a bedroom or a cooler cupboard. Always keep the inhaler in its original packaging until ready for use to safeguard against degradation due to environmental factors.
Cold-chain handling for pharmacies
For pharmacies distributing the Tiova inhaler, adherence to cold-chain logistics is vital, even though the product itself may not require refrigeration. Ensuring that the inhaler is stored within regulatory temperature ranges during transport to pharmacies helps maintain its effectiveness. Regular checks should confirm that storage conditions remain within specifications, protecting the integrity of the medication. Staff training on proper handling and storage techniques can further enhance product safety and patient outcomes.
Guidelines for Proper Use
Australian pharmacist counselling style
When counselling patients about the Tiova inhaler, Australian pharmacists typically adopt a patient-centric approach. They ensure that individuals understand the importance of adherence to prescribed dosages and demonstrate the correct inhalation technique. Pharmacists take time to explain potential side effects to watch for, such as dry mouth or throat irritation, and advise on management strategies. The style is conversational, allowing for questions and clarifications to ensure patients feel confident in managing their condition. It’s this personal touch that fosters a supportive healthcare environment, instrumental for long-term adherence.
Patient advice from PBS and national health authorities
Health authorities in Australia recommend specific practices for the effective use of the Tiova inhaler. Patients should receive clear instructions on how to use the inhaler correctly—a process that maximises medication delivery to the lungs. Regular follow-ups with healthcare providers are encouraged to assess the effectiveness and make any necessary adjustments. Importantly, authorities stress that Tiova is a long-term maintenance therapy and not intended for immediate relief during acute asthma attacks. Patients should be informed about the importance of not skipping doses and adhering to their treatment plan, promoting overall health and well-being.
| City | Region | Delivery time |
|---|---|---|
| Sydney | NSW | 5–7 days |
| Melbourne | VIC | 5–7 days |
| Brisbane | QLD | 5–7 days |
| Perth | WA | 5–7 days |
| Adelaide | SA | 5–7 days |
| Hobart | TAS | 5–9 days |
| Canberra | ACT | 5–7 days |
| Gold Coast | QLD | 5–9 days |
| Newcastle | NSW | 5–9 days |
| Wollongong | NSW | 5–9 days |
| Sunshine Coast | QLD | 5–9 days |
| Cairns | QLD | 5–9 days |
| Geelong | VIC | 5–9 days |
| Toowoomba | QLD | 5–9 days |
| Ballarat | VIC | 5–9 days |